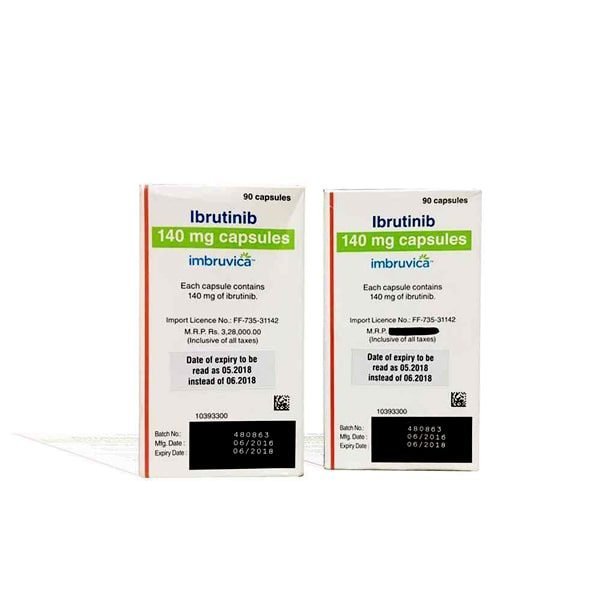
Imbruvica 140 Mg
$4,199.00 – $11,500.00
IMBRUVICA (ibrutinib) capsules for oral administration are available in the following dosage strengths: 70 mg and 140 mg. Each capsule contains ibrutinib (active ingredient) and the following inactive ingredients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate. The capsule shell contains gelatin, titanium dioxide, yellow iron oxide (70 mg capsule only), and black ink.

NHS AFFILIATED DOCTORS

FDA APPROVED PHARMACY

AFFORDABLE PRICE GURANTEE

FAST & TRUSTED DELIVERY
Imbruvica 140 Mg
IMBRUVICA (ibrutinib) capsules for oral administration are available in the following dosage strengths: 70 mg and 140 mg. Each capsule contains ibrutinib (active ingredient) and the following inactive ingredients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate. The capsule shell contains gelatin, titanium dioxide, yellow iron oxide (70 mg capsule only), and black ink.
IMBRUVICA® (ibrutinib) Structural Formula Illustration
IMBRUVICA (ibrutinib) is available as immediate-release oral capsules and immediate-release oral tablets.
IMBRUVICA (ibrutinib) tablets for oral administration are available in the following dosage strengths: 140 mg, 280 mg, 420 mg, and 560 mg. Each tablet contains ibrutinib (active ingredient) and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate. The film coating for each tablet contains ferrosoferric oxide (140 mg, 280 mg, and 420 mg tablets), polyvinyl alcohol, polyethylene glycol, red iron oxide (280 mg and 560 mg tablets), talc, titanium dioxide, and yellow iron oxide (140 mg, 420 mg, and 560 mg tablets).
Imbruvica 140 Mg is a prescription medicine used to treat adults with:
- Mantle cell lymphoma (MCL) who have received at least one prior treatment
- Chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL)
- Chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL) with 17p deletion
- Waldenström’s macroglobulinemia (WM)
- Marginal zone lymphoma (MZL) who require a medicine by mouth or injection (systemic therapy) and have received a certain type of prior treatment
- Chronic graft versus host disease (cGVHD) after failure of 1 or more lines of systemic therapy
It is not known if Imbruvica is safe and effective in children.
What are the possible side effects of Imbruvica?
Imbruvica may cause serious side effects, including:
- Bleeding problems (hemorrhage) are common during treatment with Imbruvica, and can also be serious and may lead to death. Your risk of bleeding may increase if you are also taking a blood thinner medicine. Tell your healthcare provider if you have any signs of bleeding, including:
- blood in your stools or black stools (looks like tar)
- pink or brown urine
- unexpected bleeding, or bleeding that is severe or that you cannot control
- vomit blood or vomit looks like coffee grounds
- cough up blood or blood clots
- increased bruising
- dizziness
- weakness
- confusion
- change in your speech
- headache that lasts a long time or severe headache
- Infections can happen during treatment with Imbruvica. These infections can be serious and may lead to death. Tell your healthcare provider right away if you have fever, chills, weakness, confusion, or other signs or symptoms of an infection during treatment with Imbruvica.
- Decrease in blood cell counts. Decreased blood counts (white blood cells, platelets, and red blood cells) are common with Imbruvica, but can also be severe. Your healthcare provider should do monthly blood tests to check your blood counts.
- Heart rhythm problems (ventricular arrhythmias, atrial fibrillation and atrial flutter). Serious heart rhythm problems and death have happened in people treated with Imbruvica, especially in people who have an increased risk for heart disease, have an infection, or who have had heart rhythm problems in the past. Tell your healthcare provider if you get any symptoms of heart rhythm problems, such as feeling as if your heart is beating fast and irregular, lightheadedness, dizziness, shortness of breath, chest discomfort, or you faint. If you develop any of these symptoms, your healthcare provider may do a test to check your heart (ECG) and may change your Imbruvica dose.
- High blood pressure (hypertension). New or worsening high blood pressure has happened in people treated with Imbruvica. Your healthcare provider may start you on blood pressure medicine or change current medicines to treat your blood pressure.
- Second primary cancers. New cancers have happened during treatment with Imbruvica, including cancers of the skin or other organs.
- Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure, and sometimes death. Your healthcare provider may do blood tests to check you for TLS.
The most common side effects of Imbruvica in adults with B-cell malignancies (MCL, CLL/SLL, WM and MZL) include:
- diarrhea
- tiredness
- muscle and bone pain
- rash
- bruising
The most common side effects of Imbruvica in adults with cGVHD include:
- tiredness
- bruising
- diarrhea
- mouth sores (stomatitis)
- muscle spasms
- nausea
- pneumonia
Diarrhea is a common side effect in people who take Imbruvica. Drink plenty of fluids during treatment with Imbruvica to help reduce your risk of losing too much fluid (dehydration) due to diarrhea. Tell your healthcare provider if you have diarrhea that does not go away.
These are not all the possible side effects of Imbruvica.
Read More: Imbruvica 140 Mg
Additional information
| Generic Brand | Ibrutinib |
|---|---|
| Strength | 140 mg |
| Pharma Form | Capsule/s |
| Manufacturer | Johnson & Johnson |
| Treatment | Anti Cancer |
| Pack Size | 180 Capsule/s, 270 Capsule/s, 90 Capsule/s |
| Pack Size | 90 Capsule/s, 180 Capsule/s, 270 Capsule/s |
|---|





annat –
Imbruvica 140 Mg is my lifesaving elixir. It has turned the tide in my battle against leukemia, giving me a renewed sense of life and vitality. A beacon of hope in my journey to health.